
Lithium, a.k.a. “white gold,” has never been more in demand today – this soft, silvery metal makes electric vehicle (EV) batteries store more energy and live longer. Whatever device you’re using right now to read this article is most likely powered by a lithium-ion battery. And if you are driving an electric car, this battery powers it up as well.
The demand for battery-grade lithium is projected to grow sharply in the coming years as the EV and energy storage sectors continue to rise. Lithium-ion batteries are a vital component in transitioning to a low-carbon global economy. With EVs representing 10% of total global car sales, battery makers are struggling to source more lithium.
Also, don’t forget that you can get discounted new car pricing with a free quote through qualified local dealer partners.
Lithium, the lightest of all metals, belongs to the group of elements called alkali metals. It is highly reactive and has the lowest density, making it ideal for making batteries. Dubbed “white gold” because of its soft, silvery-white appearance, lithium has been the key metal used in manufacturing rechargeable batteries, not just for EVs but also for devices like laptops and mobile phones. EV carmakers prefer lithium-ion batteries as they are lightweight and can be quickly recharged.

In the context of batteries and EVs, lithium comes in two major types: lithium carbonate and lithium hydroxide. Lithium carbonate has a wide range of industrial uses, including manufacturing EV batteries, as well as producing flooring treatments, cement densifiers, adhesives, and alloys. It is even listed by the World Health Organization as an essential medication for treating bipolar disorder. Lithium carbonate can be converted to lithium hydroxide, which is preferred for manufacturing higher-performing and longer-lasting EV batteries.
Currently, there are two major ways to extract lithium from the Earth: conventional lithium brine extraction from salt-flat brines and ore mining from lithium rock deposits. In the United States, lithium is mined from watery, salty brines called “salars.” The country currently has only one lithium mine, located in Silver Peak, Nevada. However, more refinery plants are expected to emerge as the demand for the metal soars. Tesla, for example, has broken ground at the site of its new lithium refinery in Texas, the first of its kind in North America.
The majority of commercial lithium production today comes from suppliers that extract lithium from salars, primarily in the Lithium Triangle, where Argentina, Chile, and Bolivia meet. Mining lithium from brines involves pumping salty water from underground to the surface and into a series of evaporation ponds. Over a period of up to two years, the water evaporates, and the brine changes composition, resulting in a brighter blue color. Lime and sodium carbonate are added to the brine to remove impurities and unwanted elements. Once the lithium concentration is high enough, the brine is pumped to a recovery facility for filtration of the metal. After the brine dries up, it leaves behind a white powder that undergoes further purification and chemical transformation to become a solid metal ready for use in batteries.
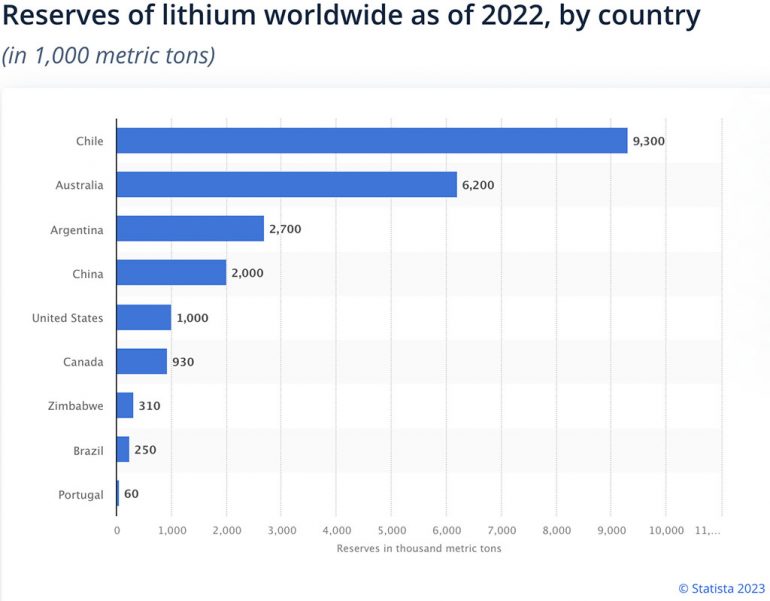
Find more statistics at Statista
In ore or hard rock mining, there is more lithium found in hard rocks compared to salty brines. However, this process requires heavy equipment and is noisy. Kings Mountain in North Carolina has some of the highest-grade lithium deposits in rocks, but its production ceased when it became cheaper to obtain lithium from brines in other parts of the country. The ore containing lithium, mainly spodumene, is extracted, crushed, and roasted at high temperatures. It is then cooled, milled, and roasted again with sulfuric acid at a lower temperature. This acid leaching process replaces hydrogen in the spodumene with lithium, resulting in the formation of lithium sulfate. The lithium sulfate is then treated with sodium carbonate, which causes lithium carbonate to precipitate out. The lithium carbonate is further processed and refined to meet the specifications required for battery-grade lithium.
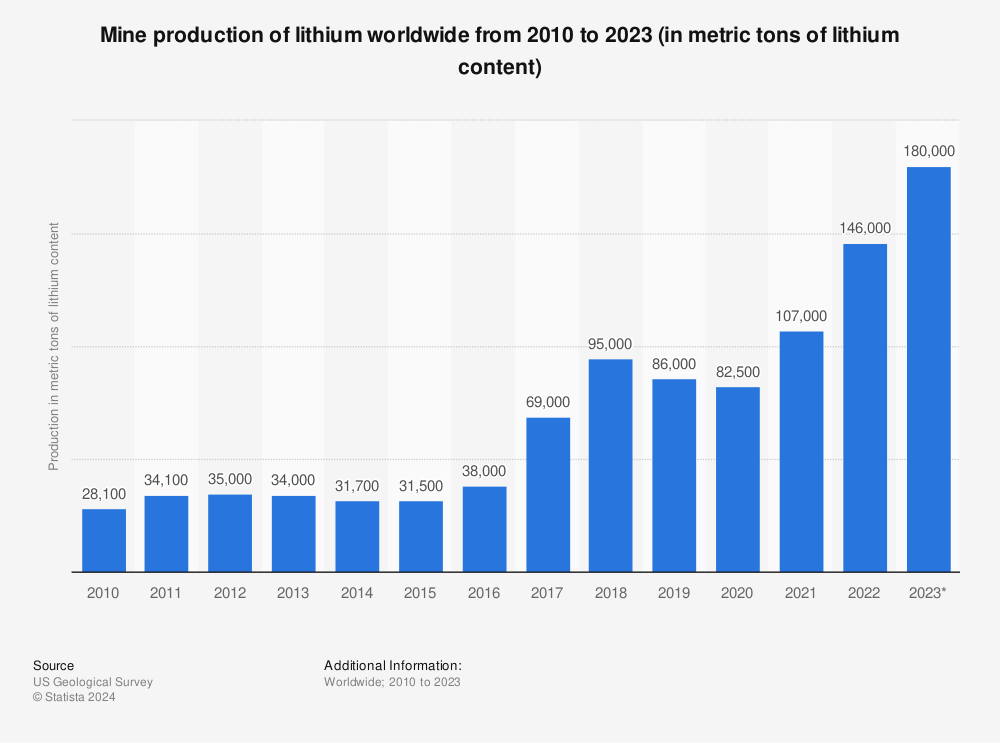
Find more statistics at Statista
Once the lithium has been extracted and refined, it can be used to manufacture lithium-ion batteries. A lithium-ion battery consists of several key components, including a cathode, an anode, and an electrolyte. The cathode is typically made of lithium compounds, such as lithium cobalt oxide or lithium iron phosphate. The anode is typically made of graphite, which can intercalate lithium ions. The electrolyte is a solution or gel containing lithium salts that allows the flow of lithium ions between the cathode and the anode.
During charging, lithium ions move from the cathode to the anode through the electrolyte, where they are stored in the anode’s graphite structure. This process is reversible, meaning that during discharge, the lithium ions move back from the anode to the cathode, generating an electric current that can power various devices, including electric vehicles.
It’s worth noting that the performance and characteristics of lithium-ion batteries can vary depending on the specific materials used and the manufacturing processes employed. Researchers and engineers are constantly working to improve battery technology to increase energy density, improve safety, and reduce costs.
As the demand for electric vehicles and energy storage continues to grow, so does the need for a stable and sustainable supply of lithium. Efforts are being made to explore alternative sources of lithium, such as geothermal brines and lithium-rich clay deposits, to diversify the supply chain and reduce reliance on traditional lithium sources.